Shaoyang Lin†, Yuval Fishler†, Soonho Kwon†, Annette Böhme, Weixuan Nie, Moon Young Yang, Jesse Matthews, Zachery W. B. Iton, Brian C. Lee, Thomas Jaramillo, Harry A. Atwater, William A. Goddard III*, Wilson A. Smith*, Kimberly A. See*, Chem. Catal., 2025, 101338, 2025, 5,6, 101338.
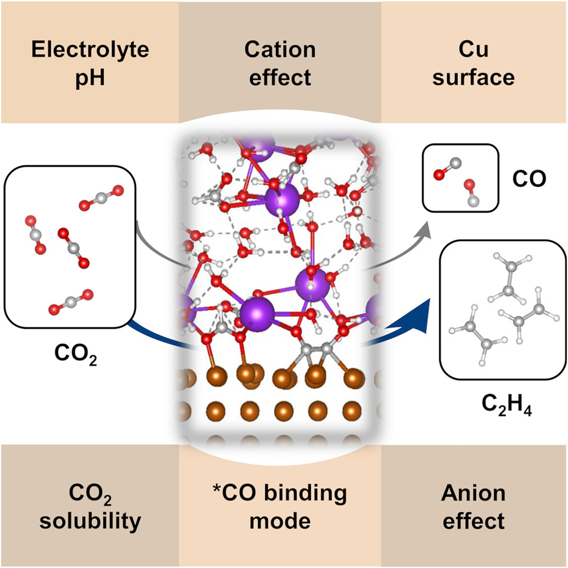
Summary: Compared to a conventional electrolyte concentration of 1 M HCOOK, the use of a highly concentrated 7.1 M HCOOK electrolyte increases the Faradaic efficiency (FE) ratio of C₂H₄/CO from 2.2 ± 0.3 to 18.3 ± 4.8 at −1.08 V vs. reversible hydrogen electrode (RHE) on a Cu gas-diffusion electrode. Based on electrochemical analysis and ab initio molecular dynamics (AIMD) simulation, the identity and concentration of the cation and anion play more important roles in controlling the CO₂R reaction pathway than the bulk CO₂ solubility and the bulk pH of electrolytes. In situ attenuated reflectance surface enhanced infrared absorption spectroscopy (ATR-SEIRAS) suggests that, unlike 1 M HCOOK, the ∗CO-bridge-binding mode on Cu is dominant in 7.1 M HCOOK electrolyte, which potentially results in less CO release and higher yield of C₂H₄. This study demonstrates that although we can tailor the electrolyte composition to shift product selectivity, the factors that control the product selectivity are numerous and cannot be distilled down into one correlated property-reactivity relationship.